ABSTRACT
Personalised medicine, often referred to as precision medicine, stands as a pivotal force in the realm of early cervical cancer detection, reshaping the landscape of healthcare. Its fundamental principle revolves around tailoring medical interventions to the specific needs of individuals, ensuring that treatments are administered with precision—the right treatment, at the right dosage, and at the right time. This approach has not only proven instrumental in saving lives but has also become a catalyst for fostering overall well-being. Cervical cancer, characterised by the abnormal growth of the cancer cells along the cervix, poses a significant threat due to its potential to metastasise to other parts of the human body. A crucial factor in the development of cervical cancer is the persistent infection of high-risk human papillomavirus (HPV), particularly strains 16 and 18. This viral connection underscores the importance of precise treatment and targeted preventive strategies. This article delves into cervical cancer, navigating its epidemiological landscape. It dissects the various aetiological factors at play while scrutinising the evolution of preventive strategies and screening protocols over time.
The United Kingdom is identified as a noteworthy focal point in this article, with nuanced initiatives implemented in the country being spotlighted. While outcomes in developed nations have been significantly improved by personalised medicine and advanced healthcare systems, a critical lens is turned toward the global scenario. In exploring current cervical screening practices, this article extends to the ethical considerations surrounding these practices in regions where economic and social factors hinder the proper care many women deserve. In essence, the complexities surrounding cervical cancer are aimed to be not only unravelled but also advocated for in a holistic and inclusive approach to screening and intervention.
Introduction
Personalised medicine, also known as precision medicine, is a medical model that considers individual variations in genes, surroundings and lifestyles to tailor the right prevention or treatment to the right person at the right time (National Human Genome Research Institute, 2023). It entails evaluating genetic data, developing personalised treatment plans, emphasising illness prevention, enhancing diagnostic precision, and optimising drug choices and dosages (National Human Genome Research Institute, 2023). Informed by the Human Genome Project (National Human Genome Research Institute), this approach enables doctors to select more precise medications and therapies tailored to a patient’s unique genetic profile, promising more effective healthcare. Cervical cancer’s ontogenesis, rooted in the abnormal proliferation of cancerous cells within the cervix, the lower part of the uterus, represents a complex and intricate process (Britannica, 2023). A large number of molecular alterations occur during the development of the malignant cells in the cervix, which lead to the unchecked proliferation of these cells. In 2020, cervical cancer ranked as the fourth most prevalent cancer among women, with approximately 604,000 new cases reported (World Health Organisation, 2023). Unfortunately, it also stood as the fourth deadliest cancer for women that year, claiming the lives of 342,000 individuals (World Health Organisation, 2023). While various factors contribute to cervical cancer, including the deleterious impact of smoking, the primary cause remains the transmission of the high-risk human papillomavirus (HPV) through sexual contact or the exchange of bodily fluids (Britannica, 2023). This article explores the role of personalised medicine in the early detection of cervical cancer through screening.
Cervical Cancer and Its Classification
Cervical cancer is the abnormal growth of cancerous cells in the cervix, the muscular ring above the vagina (Britannica, 2023). While anyone with a cervix can get cervical cancer, it is most common in people over age 30 (Centers for Disease Control and Prevention, 2023).
The anatomy of the cervix comprises two distinct parts, each covered by different cell types. The inner opening, leading to the uterus and covered with glandular cells, is termed the endocervix (American Cancer Society, 2023). The outer part, lined by squamous cells, is known as the exocervix (American Cancer Society, 2023). The meeting point of these cell types is the transformation zone, a location that may vary with age and pregnancy, and it is where the majority of cervical cancers originate (American Cancer Society, 2023). Abnormal changes in these cells, referred to as precancerous changes, gradually progress to cancer (American Cancer Society, 2023). Terms used for these changes include cervical intraepithelial neoplasia (CIN), cervical dysplasia, and squamous intraepithelial lesion (SIL) (American Cancer Society, 2023).
Cervical intraepithelial neoplasia (CIN), also known as cervical dysplasia, represents a precancerous condition characterised by abnormal cell growth on the cervix’s surface (Cleveland Clinic, 2022). CIN is categorised into three levels (CIN 1, CIN 2, and CIN 3) based on the extent of abnormal cell involvement (Cleveland Clinic, 2022). CIN 1 is less likely to progress to cancer, while CIN 2 and CIN 3 require treatment to prevent cancer development (Cleveland Clinic, 2022). Additionally, squamous intraepithelial lesion (SIL) is another precancerous condition related to abnormal tissue in the cervix, often linked to human papillomavirus (HPV) (Cleveland Clinic, 2021). SIL is classified as low-grade or high-grade, with high-grade SILs requiring immediate treatment (Cleveland Clinic, 2021).
Cervical cancer can be broadly classified into two types based on the cell where it originates: squamous cell carcinoma and adenocarcinoma (Cancer Research UK, 2023). Squamous cell carcinomas, comprising 80% to 90% of cases, develop in the exocervix’s squamous cells (Cancer Research UK, 2023). Adenocarcinomas, constituting 10% to 20% of cases, develop in mucus-producing gland cells of the endocervix (Cancer Research UK, 2023). Adenosquamous carcinoma refers to cases where both squamous and glandular cells are cancerous (Cancer Research UK, 2023). Rare types of cervical cancers include small cell cancer, lymphomas, sarcomas, and melanomas (Cancer Research UK, 2023). Cervical cancer stages range from I to IV, indicating the extent of its spread (National Cancer Institute, 2022). Each stage is further divided into subcategories based on the depth and size of the tumour (National Cancer Institute, 2022). Stage I involves cancer confined to the cervix, while stages II, III, and IV indicate increasing spread beyond the cervix, with stage IV signifying distant organ involvement (National Cancer Institute, 2022).
Table 1: The table below illustrates the stages of cervical cancer, including descriptions and subcategories (Cancer Research UK, 2023)
| Stage | Description | Substages and Details |
| I | Cancer present exclusively in cervix |
DIVIDED INTO IA & IB BASED ON:
IA DIVIDED INTO:
IB DIVIDED INTO:
|
| II | The cancer has spread beyond the cervix to the upper part of the vagina and surrounding tissues |
DIVIDED INTO IIA & IIB BASED ON:
IIA DIVIDED INTO:
IIB: The cancer has spread from beyond the cervix to tissues around the uterus |
| III | The cancer has spread far past the cervix into the lower part of the vagina, the pelvic wall, tubes (ureters) draining the kidneys, and/or to certain lymph nodes |
DIVIDED INTO:
IIIA: when the tumour has spread to the lower part of the vagina but not the pelvic wall IIB: when the tumour has spread to the pelvic wall and is blocking one or both ureters, thus affecting the kidneys IIC DIVIDED INTO:
Depending on which lymph nodes are affected, is when the tumour can be assumed to be of any size within the pelvic region, without having spread to distant areas in the body. |
| IV | The cancer has spread beyond the pelvis to the bladder, rectum, or other organs in parts of the body even farther away |
DIVIDED INTO:
Based on where the cancer has spread |
Causes and Risk Factors of Cervical Cancer
Cervical cancer, as per the World Health Organisation (2023), is primarily attributed to the persistent infection with the human papillomavirus (HPV) (Figure 1), a sexually transmitted infection. Notably, nearly all sexually active individuals will encounter an HPV infection at some point in their lives; however, in the majority of instances, the immune system clears the infection without discernible symptoms (World Health Organisation, 2023). The progression from HPV infection to cervical cancer typically involves the untreated and persistent presence of the virus in the cervix, accounting for 95% of cervical cancer cases (World Health Organisation, 2023). The evolution of abnormal cells into cancerous manifestations typically spans 15 to 20 years; nevertheless, a compromised immune system has the potential to expedite this process, reducing the timeframe to 5 to 10 years (World Health Organisation, 2023).
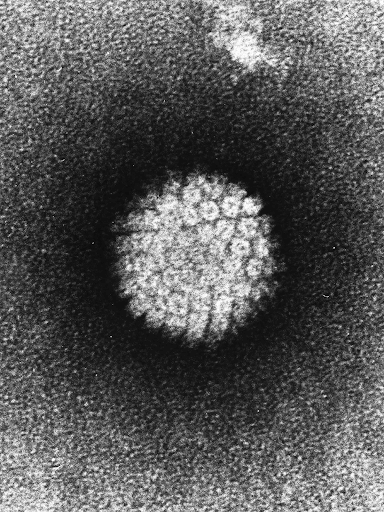
Figure 1: Electron Micrograph of the negatively stained Human Papillomavirus (HPV). (Image sourced from Britannica, 2023).
At the molecular level, the development of a cancerous cell can occur when the genetic material of the HPV virus (HPV genome) integrates into the host genome of cervical epithelial cells (Aksoy, Gottschalk and Meneses, 2017; Graham, 2017). This integration process involves the vertical transfer of genetic material, where the viral DNA becomes part of the host chromosome (Aksoy, Gottschalk and Meneses, 2017; Graham, 2017). This integration can disrupt normal cellular functions and contribute to the development of cervical cancer (Aksoy, Gottschalk and Meneses, 2017; Graham, 2017).
There is a significant correlation between the presence of tumour viruses, such as HPV, and the initiation of cervical cancer development (Senba and Mori, 2012; Balasubramaniam et al., 2019). Many individuals infected with the HPV virus exhibit diverse yet often unsuccessful immune responses in attempting to clear the virus (World Health Organisation, 2023). The persistent presence of HPV-infected cells, due to the immune system’s inability to eliminate them, heightens the risk of developing cervical cancer (World Health Organisation, 2023). Papillomaviruses harbour oncoproteins, which are encoded by oncogenes—genes with cancer-causing potential (Senba and Mori, 2012; Balasubramaniam et al., 2019). These oncogenes, when activated within host cells, can promote the growth of cancerous cells, inhibit cell death, and contribute to tumour formation (Senba and Mori, 2012; Balasubramaniam et al., 2019). The inhibition of tumour suppressors in the nucleus of cervical epithelial cells by HPV plays a pivotal role in this process (Senba and Mori, 2012; Balasubramaniam et al., 2019).
Specific oncoproteins, such as E5, E6, and E7 in papillomaviruses, exhibit conserved cancerous properties (Senba and Mori, 2012; Malla and Kamal, 2021). Upon integration into the host cell nuclei, these oncoproteins significantly disrupt the regulation of the cervical epithelial cell cycle by obstructing key tumour suppressor proteins, namely p53 and pRb (retinoblastoma protein) (Senba and Mori, 2012; Malla and Kamal, 2021). These proteins play critical roles in maintaining the cell cycle and preventing cancer development (Senba and Mori, 2012; Malla and Kamal, 2021). Furthermore, the E7 oncoproteins induce genetic instability, activating oncogenesis—the formation of tumours—and inhibiting tumour suppressor genes (Malla and Kamal, 2021). The resulting inhibition of tumour suppressants increases the likelihood of tumours in the cervix, leading to cancer.
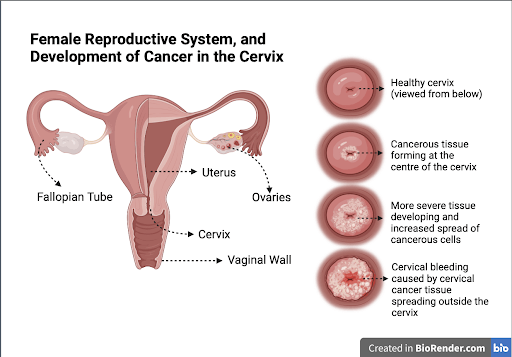
Figure 2: Depiction of cervical cancer. Illustration by Arsheya Sivakumar
In addition to HPV, numerous other risk factors contribute to the development of cervical cancer, particularly within the realm of reproductive and sexual factors. Notably, engaging in multiple sexual partnerships has been identified as a risk factor, supported by various studies indicating an elevated likelihood of acquiring HPV in individuals with multiple new sexual partners (Chelimo et al., 2013; Zhang et al., 2020). Moreover, specific research has highlighted an increased susceptibility to cervical cancer in women from the ages 20 to 31 in Germany who maintain multiple sexual partnerships (Zhang et al., 2020). Furthermore, the use of oral contraceptive pills represents another reproductive and sexual risk factor (Zhang et al., 2020). According to a study, the prolonged use of oral contraceptives for more than five years has been associated with a two-fold increase in the risk of cervical cancer (Zhang et al., 2020). This underscores the multifaceted nature of risk factors in the aetiology of cervical cancer, with both behavioural and pharmacological factors influencing the likelihood of its occurrence.
Another major contributor to cervical cancer development is exposure to components found in tobacco smoke and nicotine vapes (Castle, 2008; Qiu et al., 2016). Nicotine, a common component in tobacco smoke and vapes is known to be immunosuppressive (Castle, 2008). It increases the risk of cervical cancer in individuals infected with HPV due to its adverse effects on the robust functioning of the immune system (Castle, 2008; Qiu et al., 2016). Despite this established link, the specific molecular mechanisms underlying how smoking increases the risk of cervical precancer and cancer are not fully understood. One potential mechanism is that the harmful substances in cigarettes, such as tar, carbon monoxide, and toxic chemicals, can cause DNA damage to HPV-infected cells, elevating the likelihood of these cells becoming cancerous (Castle, 2008). Furthermore, a study found a potential link between the progression of cervical cancer and benzo[a]pyrene (BaP), a carcinogen prevalent in cigarette smoke (Alam et al., 2008). Trials demonstrated that high levels of BaP could lead to the exponential growth of the HPV virus (Alam et al., 2008). Therefore, quitting or reducing smoking can mitigate this risk.
Human Papillomavirus (HPV) Vaccination
HPV vaccines play a crucial role in preventing infections caused by the human papillomavirus, a diverse group of over 200 related viruses, with approximately a dozen having the potential to cause cancer (National Cancer Institute, 2021). The current vaccines, including Cervarix, Gardasil, and Gardasil 9, are designed based on virus-like particles (VLPs) that produce antigens which are proteins that closely mimic the structure of the virus (National Cancer Institute, 2021). For example, the L1 protein forms the surface of the HPV and the Gardasil 9 vaccine targets that and produces antigens for the body to make antibodies to immunise itself from future HPV viruses (Buck, Day and Trus, 2013). Upon administration, these vaccines stimulate the immune system to produce antibodies that bind to the HPV virus, thereby impeding its ability to infect cells (National Cancer Institute, 2021; Cancer Council, 2023a).
There exists a diverse array of over 100 HPV sub-types, each characterised by distinct mutations affecting its impact on the host cell. These types are classified based on their potential severity. Low-risk HPV strains, such as 6 and 11, lack the capability to induce cervical cancer and are more amenable to treatment (Purdie and Seladi-Schulman, 2023). Conversely, high-risk HPV strains, such as 16 and 18, pose a significant risk, with the potential to swiftly progress into malignant tumours (Purdie and Seladi-Schulman, 2023). The degree of severity associated with a specific HPV type is dependent on its viral load, representing the quantity of virus within a specified volume of fluid (Burd, 2003). For instance, HPV-16 exhibits a significantly higher viral load compared to HPV-6, rendering it a higher-risk infection (Burd, 2003).
Various vaccines have been developed to address different HPV subtypes. Cervarix targets HPV types 16 and 18 responsible for approximately 70% of cervical cancers (European Medicines Agency, 2018; National Cancer Institute, 2021). The Gardasil vaccine is effective against HPV types 6, 11, 16, and 18 (National Cancer Institute, 2021). However, it has recently been replaced by a newer version, Gardasil 9, which also includes protection against additional HPV types—31, 33, 45, 52, and 58—accounting for an additional 10-20% of cervical cancers (National Cancer Institute, 2021).
As of 2019, the World Health Organisation reported that 100 countries globally have incorporated the HPV vaccine into their national vaccination schedules. However, it is important to note that while vaccination is effective against certain HPV types associated with cervical cancer, it does not protect against all types that can cause this malignancy (Cancer Institute NSW, 2022). The recommended age for HPV vaccination is ideally before an individual becomes sexually active, aiming to prevent complications that may arise upon becoming sexually active. The Center for Disease Control and Prevention (CDC) advocates routine HPV vaccination at age 11 or 12 (Mayo Clinic, 2023). Individuals younger than 15 require two doses of the vaccine, while those aged 15 or older, or individuals with compromised immune systems, need three doses to achieve full protection (National Cancer Institute, 2021). This proactive approach aims to maximise the effectiveness of the vaccine by immunising individuals before potential exposure to the virus.
Cervical Cancer Screening
Screening serves as a pivotal process in identifying diseases or conditions in individuals before symptoms manifest (National Cancer Institute, 2023b). In the context of cervical cancer, Pap smears or tests represent a widely employed screening method (Mayo Clinic, 2023b). This procedure involves the collection of cells from the cervix, enabling the early detection of cervical cancer in women (Mayo Clinic, 2023b). The samples of cells from the cervix are preserved and sent to a laboratory to be tested for the presence of abnormal cells.
Pap smear options include liquid-based and conventional methods (Kitchen and Cox, 2023). During this procedure, a speculum is placed into the woman’s vagina to visualise the cervix (Kitchen and Cox, 2023). The liquid-based method involves collecting cells from the transformation zone of the cervix, using a brush, and transferring the cells to a vial of liquid preservative (Kitchen and Cox, 2023). Conversely, the conventional technique involves collecting cells from the transformation zone of the cervix, using a brush and spatula, transferring the cells to a slide, and fixing the slide with a preservative (Kitchen and Cox, 2023). This technique allows testing for HPV, gonorrhoea, and chlamydia from a single collection (Kitchen and Cox, 2023). Theoretically, the liquid-based technique offers advantages such as easier interpretation, fewer unsatisfactory results, and effective filtration of blood and debris (Kitchen and Cox, 2023). For women with epithelial cell abnormalities on pap smear, further evaluation is recommended (Kitchen and Cox, 2023). Colposcopy and biopsy are suggested, utilising a colposcope, which is a binocular microscope that allows visual inspection of the cervix (Kitchen and Cox, 2023). Gross abnormalities visualised on colposcopy can be biopsied for further classification (Kitchen and Cox, 2023).
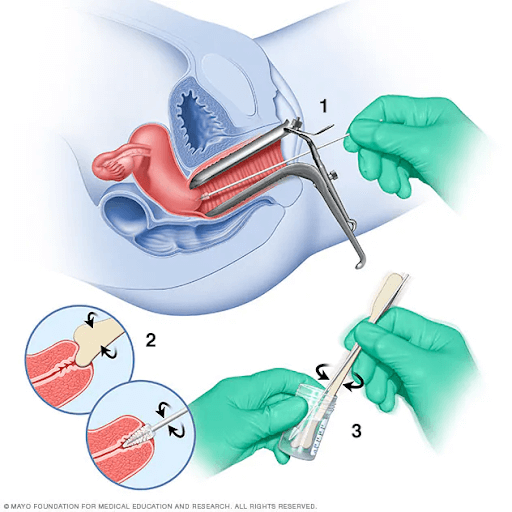
Figure 4: Depiction of a Pap smear. (Image sourced from Mayo Clinic, 2023b)
According to the Centers for Disease Control and Prevention (CDC), the effectiveness of screening methods is such that up to 93% of cervical cancers are preventable. Despite this, approximately 13,000 women are diagnosed with cervical cancer annually in the United States, with around 4,000 dying from this preventable form of cancer (Moffitt Cancer Centre, 2023). The latest recommendations from the American Cancer Society (ACS) emphasise a preference for HPV testing alone every five years for women aged 25 to 65. In the absence of this screening option, the ACS recommends a combined HPV/Pap test every five years or a Pap test every three years. For women aged 65 and older with a history of normal prior tests, no further screenings are recommended. Specific screening guidelines are outlined based on age groups. Women aged 21 to 29 are recommended to undergo a Pap test alone every three years. For women aged 25 to 29, HPV testing alone can be considered, but Pap smears are preferred. Furthermore, women aged 30 to 65 have three testing options, including Pap smears and HPV tests (co-testing) every five years.
Self-collection emerges as an alternative approach, where women take their samples for cervical screening under the supervision of a healthcare professional (Cancer Council, 2023b). The sample is obtained using a long cotton swab (Cancer Council, 2023b). A self-collected sample is taken from the vagina and is checked for HPV infection. Self-collection focuses solely on HPV detection excluding the assessment of cervical cell abnormalities. Therefore, it is typically not suitable for individuals exhibiting symptoms of cervical cancer or experiencing abnormal bleeding, pain or discharge (Healthdirect, 2023). Approximately 800 women are diagnosed with cervical cancer annually in Australia, with around 70% of cases affecting those who have not undergone regular screening or maintained up-to-date screenings (Australian Government Department of Health and Aged Care, 2023). This reveals the importance of consistent screening tests for optimal protection.
Moreover, a study assessing the reliability of real-time polymerase chain reaction (PCR) in HPV testing, a molecular technique amplifying segments of DNA, when paired with liquid-based cytology samples in cervical cancer screening has revealed that real-time PCR is a rapid and efficient method for detecting HPV (Cubie et al., 2001). Real-time PCR demonstrates the capability to distinguish between HPV-16 and HPV-18 by analysing the variance in melting temperatures (Tm) of their DNA sequences (Cubie et al., 2001). This differentiation is facilitated by their distinct responses to consensus primers, specialised DNA tools designed to target shared DNA sequences among related organisms (Cubie et al., 2001). These findings suggest the potential value of incorporating real-time PCR into cervical screening programs, offering a precise and rapid means of identifying HPV infections. It is important to highlight that the World Health Organisation (2023) recommends DNA testing as the first-line screening method for the prevention of cervical cancer.
A Personalised Approach to Cervical Screening in the United Kingdom (UK)
According to the World Health Organisation (2023), over 80% of sexually active women are projected to become infected with HPV throughout their lifetimes. In the United Kingdom, a vaccination scheme has been implemented, which has been instrumental in reducing the prevalence of HPV infections (NHS England, 2022). Notably, high vaccination coverage has resulted in a perceptible decrease in the prevalence of targeted HPV strains, having a significant impact on cervical cancer occurrence rates, as demonstrated by International Agency for Research on Cancer (IARC) statistics (IARC, 2020). Furthermore, in recognition of the association between HPV infection and cervical cancer risk, the National Health Service (NHS) in the United Kingdom (UK) has implemented a cervical cancer screening programme aimed at further lowering the risk of cervical cancer development (NHS England, 2022). The Pap smear is the principal screening technique used in the cervical cancer screening programme (NHS England, 2022). To carry out this screening, cervix cells are extracted and subsequently analysed under a microscope for any abnormalities that might point to the existence of malignant or precancerous cells. The programme involves conducting Pap smears every three years for women aged 25 to 49 and every five years for those aged 50 to 64 (NHS England, 2022).
The aim of screening is for the early identification of abnormal precancerous lesions, an area of unusual tissue that is not cancerous, but has the potential to become cancer, thereby promoting timely treatment, averting the metastasis of cancer, and enhancing overall health outcomes (NHS England, 2022). It has been observed that early detection significantly improves survival rates (National Cancer Institute, 2023a). For instance, detecting cervical cancer at Stage 1 offers approximately a 91% chance of survival, while the survival rate drops to 19% if the cancer spreads to other organs (National Cancer Institute, 2023a). This highlights the critical importance of cervical cancer screenings in enhancing both early detection and survival rates. Moreover, cervical cancer screening is instrumental in saving thousands of lives annually in the UK, preventing 70% of cervical cancer deaths in England alone (NHS England, 2022). With optimal attendance, as much as 83% of cases could be prevented (NHS England, 2022).
The UK’s cervical cancer screening programme highlights screening intervals that are customised depending on an individual’s age, HPV status, vaccination history and specific risk factors, demonstrating the dedication to precise medical practices. For instance, people with Human Immunodeficiency Virus (HIV) are deemed immunocompromised, when a person’s immune system is compromised, their body is less able to fight off infections, and therefore possess an elevated risk of cervical cancer in comparison to the general population, and would be scheduled to undergo more frequent screening for cervical cancer (NHS England, 2023).
Challenges of and Recommendations for Cervical Cancer Screening
While cervical cancer screening presents key benefits such as early detection and high survival rates, there are associated risks that should be noted. For instance, during colposcopy procedures to remove abnormal cells, some potential complications include bleeding or infection in the vagina (NHS England, 2022). Additionally, the removal of cervical tissue may impact future pregnancies, potentially increasing the likelihood of delivering a baby 1-2 months prematurely. Although this is rare, it is a possibility that must be taken into consideration (NHS England, 2022). This consideration is especially relevant for the 16% of women who undergo more intense treatments and subsequently decide to conceive (NHS England, 2022).
Personalised medicine in cervical cancer has evolved to be highly effective and tailored to each patient’s specific needs. This is evident in cervical screening practices and various treatments for cervical cancer such as immunotherapy and radiotherapy, which are customised based on individual requirements. While cervical cancer is generally preventable through early diagnosis and proper medical care, the global toll remains significant, with 300,000 deaths reported worldwide (Cohen et al., 2019; World Health Organisation, 2023). Notably, 70% of these deaths result from women not attending cervical screenings, particularly impacting regions with limited access like remote regions in Australia (National Cancer Screening Register, 2022). It is known that approximately, 90% of cervical cancer cases occur in low- and middle-income countries, such as Nepal (Cohen et al., 2019). Moreover, the challenge of ensuring the participation of all eligible individuals in the screening program can be attributed to various factors. These may include feelings of embarrassment, concerns about potential discomfort during the procedure, and a lack of awareness regarding the significance of cervical cancer screening (Healthtalk.org, 2022).
Research indicates that expanding the screening options available can effectively boost participation rates. Providing individuals with a range of screening choices empowers them to select the option that aligns with their preferences and offers the utmost comfort. A personalised approach, presenting choices like HPV tests, self-testing, and Pap smears, empowers women to select the method that aligns with their comfort and convenience, ultimately encouraging participation (Musa et al., 2017). This shift toward individualised recommendations, considering factors such as patient preferences, medical history, genetic and environmental risk factors, and interaction with the healthcare system, enhances the overall effectiveness of screening initiatives (Selby, Bartlett-Esquilant and Cornuz, 2018). In addition, it is vital to educate the individual on each option and let them make an informed decision (Musa et al., 2017). Providing support and patient education plays a crucial role in fostering comfort for patients (Devarapalli et al., 2018). A detailed explanation from the doctor regarding the screening procedure, its significance, and potential associated risks serves to alleviate anxiety and promotes attendance. Addressing individual concerns instils confidence and enhances overall comfort, as highlighted by Devarapalli et al. (2018). Recognising the uniqueness of each person’s needs and concerns is crucial, and by accommodating these factors, accessibility and effectiveness are optimised.
Another method to boost attendance to cervical screening is effective outreach strategies. This involves raising awareness through campaigns, and implementing initiatives that encourage and reassure women, providing clear explanations of the screening process, and dispelling any misconceptions. Notably, Public Health England’s (2021) national campaign, “Cervical Cancer Screening Saves Lives,” implemented during 2018-2019, has demonstrated effectiveness. Leveraging insights from this campaign can guide future interventions aimed at raising awareness about cervical cancer and promoting the benefits of screening among women. This strategic approach aligns with the evolving landscape of healthcare, emphasising tailored communication and education to enhance overall participation and outcomes in cervical cancer screening. Figure 5 illustrates a rise in the number of cervical screening samples received by laboratories in England in 2019 when compared to the figures from 2018, both before and after the implementation of the campaign (Public Health England, 2021). This suggests a positive impact of the campaign on participation and engagement in cervical screening, as evidenced by the increase in samples received by laboratories.
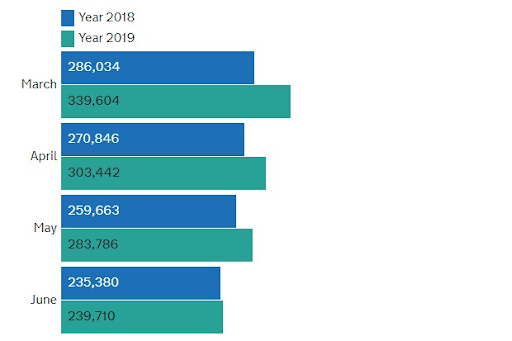
Figure 5: Bar chart representing a comparative increase in the number of cervical screening samples received by laboratories in England from 2018 to 2019 (Sourced from Public Health England, 2021).
Conclusion
In summary, cervical cancer, stemming from abnormal cell growth in the cervix and primarily associated with HPV, a sexually transmitted disease, is also influenced by lifestyle factors like smoking (Castle, 2008). Ranked as the fourth most prevalent cancer in women (World Health Organisation, 2023), cervical cancer encompasses various types such as squamous cell carcinoma and adenocarcinoma, with staging indicating the extent of spread from I to IV (National Cancer Institute, 2022). HPV vaccines, such as Cervarix, Gardasil, and Gardasil 9, play a pivotal role at the forefront of cervical cancer prevention (National Cancer Institute, 2021). Complementing these vaccines, an array of screening methods ranging from pap smears to more advanced techniques like HPV-DNA testing, further boost preventive measures and the early identification of high-risk HPV strains, and precancerous and cancerous cells (Mayo Clinic, 2023b; World Health Organisation, 2023). A personalised approach has been implemented in countries such as the UK, where the cervical cancer screening programme entails screening at specified intervals tailored to an individual’s age, HPV status, and immunocompromised condition (NHS England, 2023). Moreover, it is important to acknowledge challenges associated with low attendance in cervical screenings, often attributed to feelings of embarrassment or a lack of awareness regarding the significance of screening (Healthtalk.org, 2022). An evaluation of current screening practices has revealed areas of improvement, primarily centred around the need for increased awareness and patient education (Musa et al., 2017). Overall, the integration of personalised medicine and cervical screening has transformed cervical cancer into a treatable disease during its early stages, significantly reducing mortality rates and fostering a healthier life for many women.




